CMED publishes new regulatory framework for pricing medicines in Brazil
On December 24, 2025, the Brazilian Drug Market Regulation Chamber (“CMED”) published Resolution 3/2025 in the Federal Register, establishing criteria for the definition of prices for new products and new presentations of medicines, as provided in Article 7 of Law 10,742/2003, and setting out the procedure for filing the Price Information Document (“DIP”). Resolution 3/2025was republished onDecember 30, 2025, due to the lack of the Minister of Health’s signature. The normative text remains unchanged; only the commencement was deferred by six (6) days as a result of the republication. Accordingly, the new rules shall enter into force on April 29, 2026. Resolution 3/2025 revoked Resolution 2/2004, enacted more than two decades ago with rules focused on synthetic medicines, which therefore did not address the reality of biological and biosimilar medicines, for instance.
The final text of Resolution 3/2025 can be accessed at this link.
Hot Topics
Resolution 3/2025:
• Introduces new categories for classifying medicines with different criteria for obtaining the Factory Price (PF);
• Expands the reference basket of countries used for external price referencing. The new framework removes New Zealand from the basket and adds five (5) countries: South Africa, Japan, Mexico, Norway, and the United Kingdom;
• Expands the concept of a provisional price;
• Ties the publication of the marketing authorization to the submission of the price request (or the setting of a provisional price ex officio);
• Addresses the long‑awaited topic of incremental innovation; and
• Provides greater predictability for pricing biosimilar medicines.
On the other hand, pricing criteria for advanced therapies and radiopharmaceutical products will be addressed in a specific resolution and, for the time being, such products will be considered omitted cases. In addition, the criteria for extraordinary price adjustments for medicines were not covered in the new framework and are expected to be defined in 2026.
The resolution provides for a 120‑day transition period and, during this interval, CMED’s Executive Secretary, Mateus Amâncio, stated that CMED “will act to clarify any questions and provide the necessary guidance for a safe and transparent implementation of the new rules.”
I. Preliminary Provisions of the New Framework
Resolution 3/2025 standardizes the concept of incremental innovation as a change relative to an originator medicine resulting from innovative activity, not considering as such mere variations in simple product characteristics, such as:
(i) purely aesthetic changes to the product;
(ii) routine or insignificant changes in the product’s functions or characteristics that do not involve sufficient novelty or technological effort and do not add anything significant to its performance;
(iii) changes to the product name or to the size or volume of the packaging;
(iv) commercialization or manufacturing of new products fully developed and produced by another company;
(v) customization for a client, that does not include significant differences in attributes when compared to products registered by other companies in the country.
In this sense, the new framework also introduces the concept of a medicine with incremental innovation as a medicine that demonstrates innovative activity in relation to an originator medicine already registered in the country, consisting of a new combination, new monodrug, new route of administration, new strength, new dosage form, new packaging, or other incremental innovation, according to the following definitions:
(i) new combination: a medicine with a new combination of two or more active pharmaceutical ingredients (“APIs”) in the country, of medicines already registered and active in CMED’s database, including fixed‑dose combinations;
(ii) new monodrug: a medicine with only one API from a combination previously registered in the country and active in CMED’s database;
(iii) new route of administration: a medicine with a new route of administration in the country that has the same dosage form, same strength, and the same therapeutic indication in relation to an originator medicine currently active in CMED’s database;
(iv) new strength: a medicine with a new strength in the country that has the same dosage form in relation to an originator medicine currently active in CMED’s database;
(v) new dosage form: a medicine with a new dosage form in the country in relation to an originator medicine currently active in CMED’s database;
(vi) new packaging: a medicine with new packaging in the country that has the same dosage form, same strength, and the same therapeutic indication in relation to an originator medicine currently active in CMED’s database;
(vii) other incremental innovation: medicines with incremental innovations other than those defined in the concept of a medicine with incremental innovation in relation to an originator medicine already registered in the country, including a new administration device.
i. New chronological sequence for price submission
The new framework determines that holders of the marketing authorization must submit a price request to CMED after submitting the marketing authorization application and before its publication. It will be the company’s responsibility to monitor the progress of the marketing authorization process with Anvisa and take the necessary steps to comply with Article 6 of Resolution 3/2025. If the marketing authorization holder does not submit the DIP by the time Anvisa publishes the marketing authorization, CMED must initiate ex officio proceedings to define the medicine’s Factory Price (“PF”).
This obligation was included in the regulation to comply with what was determined by the Federal Supreme Court under Theme 1234.
ii. Pre‑submission meeting
Resolution 3/2025 provides that interested parties may request a pre‑submission meeting with CMED for the presentation of the DIP.
iii. Criteria for Price Determination
- External price referencing: The PF proposed by the company may not be higher than the lowest PF practiced for the same product in reference countries, with applicable taxes added.
- Fourteen (14) reference countries, the so‑called “basket of countries”: South Africa, Germany, Australia, Canada, Spain, the United States of America, France, Greece, Italy, Japan, Mexico, Norway, Portugal, and the United Kingdom, in addition to the product’s country of origin.
- The new framework removes New Zealand from the basket and adds five (5) countries: South Africa, Japan, Mexico, Norway, and the United Kingdom.
- Definitive Price and Provisional Price:
- For the PF to be calculated, the product must be marketed in at least four (4) basket countries, and the price sources must be publicly available for consultation;
- If the product is not marketed in at least four (4) basket countries, CMED will set a provisional price for the product, and the company must submit to CMED, annually, a document proving the product’s launch and its respective price in the reference countries;
- The provisional condition does not apply to new products developed and manufactured in Brazil;
- To convert a foreign currency price into the national currency, the average selling exchange rate published by Brazil’s Central Bank for the 60 business days prior to the date of approval of the Technical Opinion, or to the appeal decision date, as applicable, will be used.
- The company may request, up to the first‑instance decision by CMED, an update of the price sought in the event of significant appreciation or depreciation of the exchange rate, without prejudice to the analysis period.
iv. Categories of Medicines and Criteria for Setting the PF by Category
The table below summarizes the categories of medicines provided in the new framework and the respective criteria for defining the PF by category, without prejudice to the detailed explanation throughout the text:
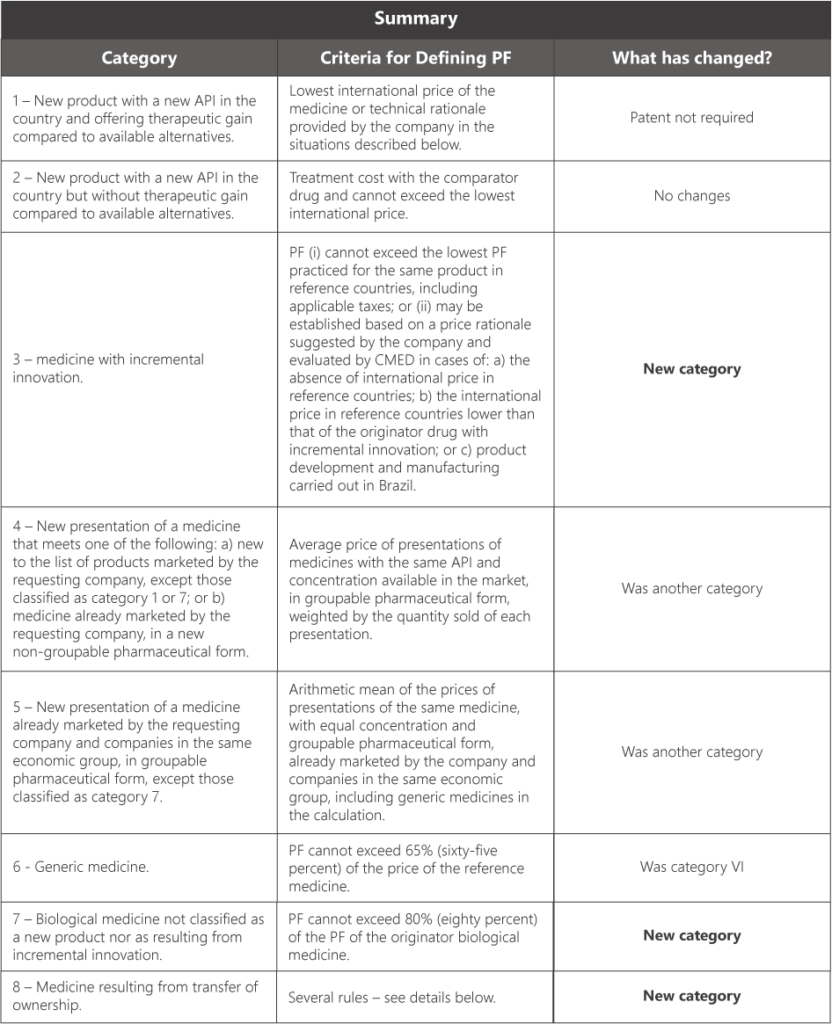
The new framework provides eight (8) categories for classifying new products, namely:
- Category 1: new product that has a new API in the country and demonstrates therapeutic gain compared with the therapeutic alternatives available in the country. A patent on the molecule is no longer required to qualify for this category. New presentations of medicines classified in Category 1 that are later launched on the market will follow, for a period of five (5) years, the same categorization.
- Category 2: new product that has a new API in the country and does not demonstrate therapeutic gain compared with the therapeutic alternatives available in the country.
- Category 3: medicine with incremental innovation, according to the following types: (a) new combination; (b) new monodrug; (c) new route of administration; (d) new strength; (e) new dosage form; (f) new packaging; or (g) other incremental innovation.
- Category 4: new presentation of a medicine that falls under one of the following situations: (a) new to the list of products marketed by the requesting company, except those classified as category 1 or 7; or (b) medicine already marketed by the requesting company, in a non‑groupable dosage form.
- Category 5: new presentation of a medicine already marketed by the requesting company and by companies in the same economic group, in a groupable dosage form, except those classified as category 7.
- Category 6: generic medicine.
- Category 7: biological medicine that is not classified as a new product nor as resulting from incremental innovation. Biosimilars fall under this category.
- Category 8: medicine resulting from a transfer of ownership.
To detail the table above, Resolution 3/2025 provides the following criteria for setting the PF:
- Category 1: the lowest international price of the medicine or a technical rationale submitted by the company, to be assessed by CMED in the following situations: (i) the lack of an international price in the reference countries; or (ii) the product’s manufacturing process and its development being carried out in Brazil. In the latter case, CMED must consider, among other elements, the degree of additional benefit provided by the medicine and the degree of innovative activity undertaken by the company in the country for the development and production of the medicine.
- Category 2: the treatment cost of the comparator medicine, which may not be higher than the lowest price practiced among the reference countries. In this case, the treatment cost of the product classified in Category 2 shall also not be higher than the treatment cost of the medicine chosen as the comparator.
- Category 3: the PF (i) may not be higher than the lowest PF practiced for the same product in the reference countries, with applicable taxes added; or (ii) may be set based on a price rationale suggested by the company and assessed by CMED in the following situations: (a) the absence of an international price in the reference countries; (b) an international price in the reference countries lower than that of the originator medicine with incremental innovation; or (c) the product’s manufacturing process and development being carried out in Brazil. The PF of a medicine with incremental innovation may not be lower than the PF of the originator medicine with incremental innovation.
- Category 4: the PF will be defined based on the average price of medicine presentations with the same API and the same strength available on the market, in a groupable pharmaceutical form, weighted by the quantity sold of each presentation, based on the following: (i) the weighted average must be calculated based on presentations of equal strength and groupable pharmaceutical form existing on the market, provided that the groupable forms do not have different posology; and (ii) if there are no presentations of equal strength, the weighted average must be calculated based on all presentations with the same API and groupable pharmaceutical form existing on the market, according to the criterion of direct proportionality of the API strength, provided that the groupable forms do not have different posology.
- Category 5: the PF will be defined based on the arithmetic average of the prices of presentations of the same medicine, with equal strength and groupable pharmaceutical form, already marketed by the company itself and by companies in the same economic group, with generic medicines being included in the calculation.
- Category 6: the PF may not be higher than 65% (sixty‑five percent) of the price of the reference medicine. The PF will be calculated considering the PF allowed for the reference medicine at the time of its entry into the national market, updated by the annual adjustment indices permitted by Law 10,742/2003.
- Category 7: the allowed PF may not be higher than 80% (eighty percent) of the PF of the originator biological medicine. The allowed PF will be defined according to the criterion of direct proportionality of the API strength of the originator medicine when there is no originator medicine of equal strength, applying a 20% (twenty percent) discount.
- Category 8: the PF will be defined according to the following criteria: (i) if the successor company does not have in its portfolio a presentation of a medicine with the same API, strength, and groupable dosage form, the price of the presentation whose registration ownership has been transferred may not be higher than the PF of the presentation of the former holder of the registration; (ii) if the successor company already has in its portfolio a presentation of a medicine with the same API, strength, and groupable dosage form, the allowed PF may not be higher than the arithmetic average of the prices of the respective presentations of the medicines of the current holder, with generic medicines being included in the calculation, nor higher than the PF of the presentation of the former holder of the registration. If it is a transfer of ownership of a generic medicine, only presentations of generic medicines will be considered; if it is a transfer of ownership of a reference medicine, only presentations of the reference medicine will be considered. Retroactive price adjustment will not be granted, except when the presentation of the succeeded company is inactivated in CMED’s database, in which case the new holder will be entitled to the adjustments applied after the date of inactivation.
v. Marketing Authorization Conditional Upon Submission of Additional Data and Evidence & Provisional Price
CMED may set a provisional price in cases where the marketing authorization is conditional upon the submission of additional data and evidence after the authorization has been granted, when the information necessary for the product’s definitive pricing is not yet available at the time the DIP is submitted.
In such cases:
- CMED’s decision must specify the additional data and evidence whose submission will be considered essential for setting the definitive price; or
- CMED may decide that it is necessary to await full compliance with the commitment term signed with Anvisa in order to establish the definitive price.
- Until the definitive price is set, the company must submit to CMED the Technical Report with data showing evidence of the product’s efficacy and safety whenever such data are submitted to Anvisa’s marketing authorization area, according to the schedule set out in the commitment term signed for the product’s authorization.
- CMED must set a definitive price for these products within 90 (ninety) days from the date of full compliance with the commitments undertaken with Anvisa or from the date on which the information specified in CMED’s decision is provided. If CMED does not issue a decision within this period, the medicine must be marketed at the provisional price until the decision is communicated.
vi. Documents in a Foreign Language
Documents accompanying the DIP must be submitted in Portuguese, but the new framework also states that documents in English and Spanish will be accepted.
If translation of documents originally submitted in English and Spanish is necessary, a request for clarification will be issued asking for the translation of the documents. Where a translation is required, and in the absence of a specific rule requiring sworn translation, a free (non‑sworn) translation may be accepted.
Documents accompanying the DIP, when submitted in a language other than Portuguese, English, or Spanish, must be submitted via sworn translation referring to the identification of the price or to the lack of a price in the reference countries.
vii. First‑Instance Decision and Deadlines
CMED’s Executive Secretariat (“SCMED”) is responsible for issuing first‑instance decisions on price requests for new products and new presentations submitted, and must observe the following deadlines, counted from the date of publication of the marketing authorization:
- up to 60 (sixty) days for products classified in categories 4, 5, 6, 7, and 8; and
- up to 90 (ninety) days for products classified in categories 1, 2, and 3 or as omitted cases.
These deadlines may be extended once, for an equivalent period. In addition, SCMED must prioritize the analysis of DIPs whenever there is a formal and duly substantiated request from the Ministry of Health.
viii. Administrative Appeal
An appeal may be filed with the Technical‑Executive Committee (“CTE”) against SCMED’s decision within 30 (thirty) days from the notification of the decision. The appeal will be addressed to SCMED, which may reconsider the first‑instance decision within 90 (ninety) days.
If there is no reconsideration of the decision, or upon expiry of the reconsideration period, the case will be sent to the CTE for a decision on the appeal. If SCMED’s reconsideration only partially accepts the grounds of the appeal, the company will be notified so that, if it wishes, it may file an appeal with the CTE within 30 (thirty) days from the notification.
ix. Mandatory Reexamination
SCMED decisions will be subject to mandatory reexamination by the CTE when the company does not file an appeal, in the following cases:
- omitted cases, except for situations supported by a CTE statement; and
- when the maximum PF permitted for the medicine is defined on the basis of Article 16 (Category 1, based on the pricing rationale proposed by the company), item II of Article 18 (Category 3, based on the pricing rationale proposed by the company), or Article 19 (Category 3 when the medicine provides an additional benefit, has its production process and development carried out in Brazil, and falls within a relevant market with a Herfindahl–Hirschman Index (HHI) below two thousand five hundred), calculated per API and considering only companies from distinct economic groups.
The CTE’s decision in the mandatory reexamination may confirm the first‑instance decision or modify it to set a higher or lower price than that calculated by SCMED. In situations where the mandatory reexamination results in the definition of a price lower than that established by SCMED, a request for reconsideration may be filed with the CTE itself within 30 (thirty) days, and the case will be assigned to a new rapporteur.
CMED may review its decisions when it identifies:
(I) at any time: (i) an error or inaccuracy in the information submitted by the applicant company, when bad faith is proven, without prejudice to any applicable sanctions; or (ii) material inaccuracies or calculation errors, ex officio or upon request by the party; or
(II) an error in the assessment of the documentation at any of its decision‑making levels, by means of self‑review, within five years from the start of commercialization.
X. Disclosure of Prices
CMED decisions on DIPs, at any level, will result in the disclosure of the approved prices in a list published monthly on its website and on Anvisa’s website, which may be amended if the decision is modified in reconsideration or appeal proceedings. The price list published on Anvisa’s website will include an indicator showing cases pending judgment by CMED.
XI. Final and Transitional Provisions
The PF obtained from the calculations provided for in the new framework will be expressed with two decimal places, with rounding from the third decimal place, as set out in item “7. Rounding of Numerical Data” of the publication Standards for Tabular Presentation of the IBGE (Brazilian Institute of Geography and Statistics).
Failure to comply with the provisions of Resolution 3/2025 will subject the violator to the sanctions set out in Law 10,742/2003. Doubts arising from the application of Resolution 3/2025 will be resolved by the CTE, after hearing SCMED.
XII. Five‑Year Review of the New Medicine Pricing Framework
The CTE will periodically coordinate evaluation processes of Resolution 3/2025, observing good regulatory practices, to assess the need for its amendment by the Council of Ministers. The evaluation processes will be carried out at intervals no longer than five (5) years.
XIII. Omitted Cases: Advanced Therapies and Radiopharmaceutical Products
The criteria for setting prices for advanced therapy products and radiopharmaceutical products will be regulated in specific acts of the Council of Ministers. Thus, advanced therapy products and radiopharmaceutical products will be considered omitted cases until the specific acts of the Council of Ministers are issued.
XIV. Transition Regime
The new regulatory framework for medicine pricing applies to:
- DIP analyses pending first‑instance judgment at SCMED;
- Products classified as omitted cases that are before the CTE for first‑instance judgment; and
- Products that have provisional prices established under Resolution 2/2004 and that have not yet become definitive.
Companies requesting pricing for medicines that fall under the situations above must submit the supplementary documentation required by Resolution 3/2025 within 30 (thirty) days from its effective date. In cases where the supplementary documentation is not submitted within the specified period, SCMED must notify the applicant company to comply with the determination within 30 (thirty) days. Failure to observe the 30‑day period will result in the initiation of ex officio proceedings to define the initial PF for the medicine.
Administrative appeals and mandatory reexaminations that are under review at the CTE, as well as appeals pending judgment by the Council of Ministers, will continue their course before those instances until judgment, observing the rules set out in Resolution 2/2004.
XV. Revoked Rules
The following Resolutions are revoked:
- Resolution 2/2004;
- Resolution 4/2005; and
- Resolution 4/2006.
The Life Sciences & Healthcare team at Souto Correa is available to answer any questions about the new medicine pricing framework, its transition period, and its possible developments at lifesciences@soutocorrea.com.br.