Regulation of the Clinical Research Law and next steps
On October 8, 2025, Decree No. 12,651/2025 came into force, regulating Law No. 14,874/2024 and establishing the National System of Ethics in Research with Human Subjects (SNEP). The regulation consolidates guidelines for ethical approval, governance, and accreditation of Institutional Review Boards (IRBs, known as CEPs in Portuguese), bringing greater legal certainty to the sector.
Topics Not Fully Addressed in the Decree
Some matters provided for in Law No. 14,874/2024 are not deeply addressed in the Decree and will require further regulation, such as: complementary guidelines on post-trial access; creation of a national registry of clinical trials; biobank and biorepositories. These will be addressed after the establishment of the National Ethics Authority (INAEP).
Structure of the National System and Its Multidisciplinary Composition
The Decree sets forth the structure of the SNEP, composed of two bodies:
- National Ethics Authority (INAEP): A collegiate body linked to the Ministry of Health, with normative, supervisory, and appellate functions.
- Ethical Review Instance: Represented by CEPs, with consultative and deliberative roles, acting independently to review and approve protocols and monitor study execution.
The multidisciplinary composition of INAEP, with 33 members, is a positive aspect, especially the inclusion of 15 experts with recognized knowledge and relevant experience in ethics in research with human subjects, selected through:
- A public process based on criteria of regional, ethnic-racial, gender, and interdisciplinary representation.
- Requirements include a Ph.D. or at least ten years of experience in IRBs or in drafting, reviewing, and conducting research protocols.
- Terms will last three years, with possible renewal subject to performance evaluation.
CEP Accreditation and Scope of Review
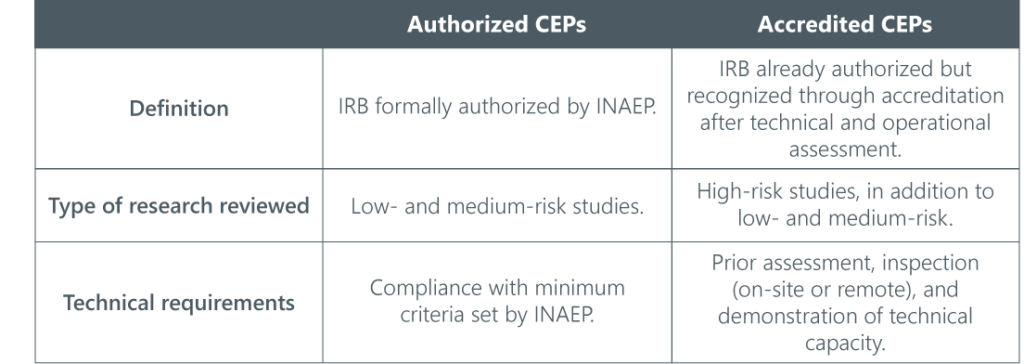
This classification will be regulated by INAEP and may include on-site or remote inspections, based on objective criteria, with prioritization possible for public interest or health emergencies.
Multidimensional Risk Classification
Ethical review will be guided by a multidimensional risk classification, essential to define the competent IRB and applicable procedures.
Criteria for classifying the degree of risk include: Nature of intervention or procedure; degree of invasiveness and potential harm; population involved, with special attention to vulnerable groups (children, pregnant women, indigenous peoples, persons deprived of liberty, persons with disabilities); use of emerging technologies, sensitive data, or AI in health; scientific uncertainty regarding study effects; direct benefits to participants and society; methodological complexity; clinical development stage of the product or technology; and multicentricity or internationalization of the research.
Depending on the classification, simplified procedures for submission, review, and authorization may apply, and protocol processing will vary according to risk level.
Post-Trial Access: Guarantee of Therapeutic Continuity
The Decree mentions post-trial access but leaves detailed regulation of drafting, submission, and ethical review of post-trial supply plans to INAEP.

The sponsor must ensure free supply of the investigational product after the end of the clinical trial whenever it is considered the best therapeutic alternative.
Priority for Strategic Research for SUS
The Decree provides mechanisms to expedite ethical review of studies considered strategic for SUS, technological innovation, and response to health emergencies, which will have priority processing, with a maximum period of 15 days for ethical review.
National Platform for Research with Human Subjects
The Decree regulates the creation of an integrated electronic platform, which will be maintained by the Ministry of Health and will have the following functionalities:
- Registration of studies and volunteers;
- Electronic submission and tracking of documents;
- Secure communication between sponsors, researchers, and regulatory bodies;
- Protection of sensitive data and trade secrets;
- Public database, updated and accessible.
This centralization represents an important advance in regulatory efficiency, transparency, and legal certainty for clinical trials in the country.
Next Steps: Implementation and Regulation
The Decree provides for the creation of a temporary working group, with an initial duration of three months, coordinated by the Secretariat of Science, Technology, and Innovation of the Ministry of Health. This group will be responsible for:
- Drafting the internal regulations of the National Instance;
- Proposing complementary rules on accreditation, biobanks, vulnerable groups, human sciences, among others;
- Procedures for integrated ethical review with ANVISA; and
- Presenting a technical report with justifications for the proposals.
Meanwhile, the rules of the National Health Council that do not conflict with the Law or the Decree remain valid. In addition, CEPs already accredited and certified under the current system maintain this status until future evaluation by INAEP.
The regulation has arrived, but the system is still under construction. Our Life Sciences team will closely monitor the next developments in processes and preparation for a more structured, demanding, and transparent ethical environment.
The Life Sciences & Healthcare team at Souto Correa is available should you have any questions about the topics and their possible developments via email: lifesciences@soutocorrea.com.br.
